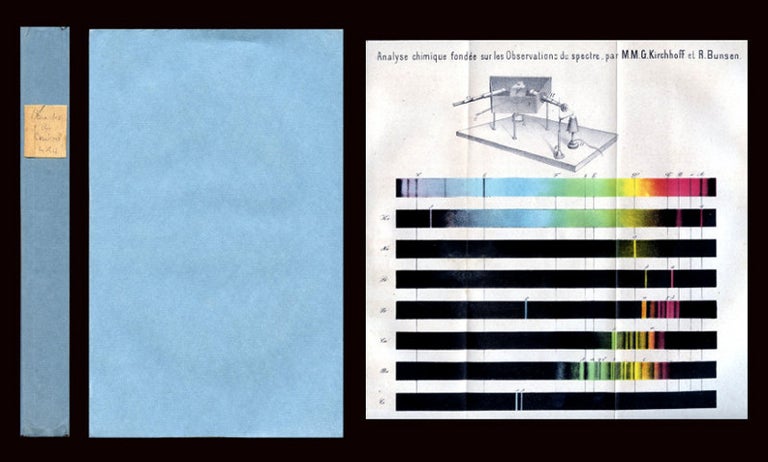
Analyse chimique fondée sur les Observations du Spectre. Premier Mémoire, in Annales de Chimie et de Physique 3rd Series, Volume 52, 1861.
1861. 1st Edition. FIRST EDITION and appearance in French of an 1861 landmark paper marking "the invention of Spectral Analysis and the announcement of the discovery of two new elements, Cesium and Rubidium [and eventually many more] by using the new method of spectroscopy developed by Kirchhoff and Bunsen.
The spectral lines that Kirchhoff and Bunsen discovered "predicted and demonstrated that new elements, so far missing in the Periodic Table, could be found with the help of spectral analysis. The spectra of the elements would later yield most important information about the structure of the atoms and would help to explain the Periodic Table" (Brandt, The Harvest of a Century, 28).
Kirchhoff and Bunsen’s discoveries were heralded as "One of the most dashing advances of the human mind into the secrets of the composition of matter on earth and in cosmos"(Kedrow, Spektralanalyse, 1961). Kirchhoff and Bunsen’s collaboration would change the world, not just of chemical analysis, but also of astronomy and physics. Working with Bunsen at Heidelberg, Kirchhoff made a completely unexpected discovery. It was known at the time that, if held to flame, many chemical substances colored the flame in consistent, characteristic ways. Kirchhoff, without intending to, had identified the cause of the dark lines seen in the solar spectra by Fraunhofer and others. When certain chemicals were heated in Bunsen's burner, characteristic bright lines appeared, i.e., elements in the substance. In some cases these were at exactly the same points in the spectrum as Fraunhofer's dark lines. The bright lines were light coming from a hot gas, whereas the dark lines showed absorption of light in the cooler gas above the Sun's surface.
The two scientists found that every chemical element produces a unique spectrum. This provides a sort of "fingerprint" which can confirm the presence of that chemical. Kirchhoff and Bunsen’s worked out the laws of spectroscopy: "(1). An incandescent body gives off light in a continuous spectrum. (2) An excited (heated) body gives off a bright-line spectrum. (3) A vapor of an element absorbs white light in the places of the spectrum (producing dark lines) at the same points where the element heated to incandescence produce bright lines" (Ede, The Chemical Element: A Historical Perspective, 75). Item #107
CONDITION & DETAILS: Entire volume. 8vo (9 x 5.5 inches; 225 x 138mm). 512 pp. Kirchhoff & Bunsen paper: 452-486. Illustration: 1 double-page folding chromolithographed plate depicting spectrum analysis (the colors remain very bright); another plate shows a spectroscope and 8 spectra, among these the spectra of Cesium. Binding: Entire volume in blue paper wraps with a chipped label at the spine. The wraps are in excellent condition and the text block is solid and tight. Interior: Complete and uncut. Some light foxing and toning throughout as well as a light (and minor) old damp stain at the margin, otherwise clean throughout.
Price: $500.00